Among the cells of our immune system, the B cells make our antibodies. Each B cell makes a different antibody. We make an estimated one trillion different antibodies, giving us the capacity to respond to virtually any pathogen.
Even more remarkably, each of these antibodies reacts with a variety of different antigens (an antigen is something that produces an antibody response) with relatively low specificity and affinity (stickiness) at first. Once we are exposed to a pathogen, we begin making highly specific antibodies that are incredibly “sticky” towards their antigens.
Thus, while we might make a trillion different types of antibodies at any given time, over our lifetime we make far more different types of antibodies than that.
But wait! Antibodies are proteins, and proteins are encoded by genes. We only have about 25,000 genes, so how on earth can we make trillions of different antibodies?
Moreover, how can the nature of these antibodies change after we are exposed to pathogens?
The answer is fascinating for two reasons. First, there exists within our lymph organs a microcosm of Darwinian evolution by natural selection where we see generations upon generations of evolution within a single immune response. Second, we see a rather “intelligent” response of the cell where it actually deliberately creates its own antibody gene in order to make a “perfect fit” for the antigen it encounters.
What follows comes from chapter 25 of the most recent edition (2008) of Molecular Biology of the Cell. Since they have scary copyright warnings, however, the images are my own creations.
Most of the research that is discussed in this post was the basis for Susumu Tonegawa's reception of the 1987 Nobel Prize in Medicine.
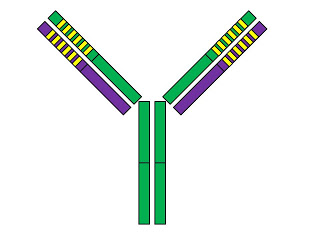
First, let's take a look at the basic structure of an antibody:
The antibody is Y-shaped and consists of four protein chains. Two of them, shown in green, are called “heavy chains,” while the other two, shown in purple, are called “light chains.” The solid colors show the “constant” regions of these chains while the yellow stripes mark the “variable” regions.
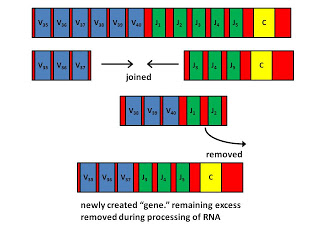
The variable regions are what gives antibodies their diversity. Each B cell creates its own antibody gene that has a unique variable region by assembling different “gene segments” together. This is called combinatorial diversification, and is illustrated in the following diagram.
The above diagram shows part of a coding region for a human light chain. There are forty V (variable) segments, which are shown in blue, but most of which are not shown. There are five J (joining) segments shown in green, and one C (constant) segment shown in yellow.
In between the V segments and in between the J segments there are “recombination sequences.” These are specific DNA sequences that tell our enzymes where to cut the DNA. The recombination sequences between V segments are different from those between J segments, so that the cell never makes a mistake by combining two V segments or two J segments together.
Two proteins called RAG1 and RAG2 combine to make the RAG complex, which is like a pair of scissors, and is only found in our B cells and T cells. The RAG complex cuts out a region between the chosen V segment and the chosen J segment. Then, the ordinary DNA repair enzymes that are found in all of our cells engage in a process called site-specific recombination in order to join the DNA back together. During this joining, a few nucleotides are allowed to fall off and are often replaced with different nucleotides, allowing further diversification. The cell has now created its own antibody gene.
During this process, the cell also edits the DNA surrounding this new “gene” so that specific DNA sequences called promoters and enhancers are positioned correctly to allow the gene to be expressed. Thus, a B cell can only make an antibody after it engages in this DNA-editing, gene-creating process.
There are some excess V and J segments left over that flank the desired portion of the gene. When the cell uses the gene template to make an RNA molecule that will later serve as a template to make the final protein, this extra portion of the RNA is removed.
For heavy chains, the process is similar, except that heavy chains also have a set of 25 D (diversity) segments,and have six J segments instead of five, and have five different C segments that can be selected depending on whether the B cell will specialize in making IgM, IgD, IgE, IgA, or IgG antibodies, all of which would bind to the same antigen but each of which has unique functions that determine what the antibody does with the antigen it has bound.
This process of combinatorial diversification is also called V(D)J joining. This allows B cells to make hundreds of different light chains and thousands of different heavy chains. The same basic process underlies the production of T cell receptors in T cells.
However, B cells undergo a further process that T cells do not, called somatic hypermutation. This occurs in lymph follicles after B cells have been activated by antigens and “helped” by helper T cells. The B cells form clusters where they reproduce rapidly but deliberately ramp up their mutation rate in these variable regions to a rate that is one million-fold higher than the normal rate.
They express antibodies on their cell surface that act as “receptors” for antigens. The cells that do not bind well to the antigens commit suicide, while the cells that bind the best proliferate rapidly. This process is called affinity maturation and is responsible for the increasing immunity we receive after we have been exposed to a pathogen.
This is rather remarkable in that it seems to be a microcosm of Darwinian natural selection.
Only there are several important differences. First, the B cells that bind poorly to the antigen are not slayed by starvation or inability to find a mate as they are out-competed by their more successful brethren. Instead, they sacrifice themselves and commit suicide. Second, there is a clear communication of binding affinity for the antigen from the cell surface to the B cell's genes. These genes altruistically commit suicide only after receiving this communication. Third, the mutation rate is deliberately increased one million-fold by a special enzymatic process.
In this process, an enzyme called “activation-induced deaminase” converts cytosine to uracil. Since uracil does not belong in DNA, the DNA repair enzymes replace it with legitimate nucleotides. This special mutational process happens only in the V regions of antibody genes, so the mutation is not truly “random.”
Thus, while there are superficial similarities to Darwinian concepts of evolution by natural selection of random variation, the process is more indicative of a natural genetic engineering within the cell.
We must then deal with the problem of autoimmunity. After all, if we can produce trillions of antibodies and respond to practically anything, why don't we usually destroy our own tissues? The first defense against this danger is called “receptor editing.” B and T cells that react to our own tissues undergo a second round of V(D)J joining in order to further edit the antibody/T cell receptor genes. If they are still self-reactive, they will either be destroyed, permanently inactivated, or indefinitely suppressed. The eventual failure of this suppression in some cases is believed to be related to autoimmune disorders.
How do B and T cells tell the difference between antigens they should destroy and those they should not? They require signals to interpret whether the antigen they bind to is a “good guy” or “bad guy.” Scientists debate whether these signals result from certain molecular patterns that look like “pathogens,” or instead from patterns that indicate general “danger,” whether they originate from pathogens or our own body. But when all is in working order, we regulate our vast array of immune potential to use it to our advantage.
Of course, there may still be a degree of “genetic determinism” to our immune response. While our B cells can make antibodies to any antigen and our T cells can make T cell receptors for any antigen, important cells called antigen-presenting cells do not have that luxury. They have a number of major histocompatibility complex (MHC) proteins, which are called human leukocyte antigens (HLA) in humans. These are thought to be limited because if they were not, a great number more T and B cells that are otherwise useful would become self-reactive and we would have to delete them. This would compromise our defense against pathogens rather than boost it.
Antigen-presenting cells use these proteins to present digested fragments of antigens to T cells. The T cell is then activated, and corresponding helper T cells “help” B cells to become activated and start making antibodies. Since the antigens are digested into many fragments, and since we generally have 12 or more different HLA proteins, at least one of these proteins is usually able to present at least one of these fragments to a T cell. Thus, we can respond to virtually any pathogen.
However, many scientists believe that once in a great while when an epidemic sweeps through a population killing many people, it is because most of the people in that population did not have the right MHC proteins to handle that pathogen's unusual protein fragments. And some scientists also believe we are attracted to people with different HLA genes than we have precisely so our children will be sure to have a diverse array of them and thus be maximally resistant to pathogens.
Fascinating? I think so. But let me know what you think in the comments.
New listener/subscriber. Was wondering if you’ve done anything newer on Autoimmunity? Recently discovered that my IBS appears to be related to the auto antibodies I have for vinculin. Look up IBSSmart if this is something your not familiar with, guessing you are though.
The test was pretty informational as far as knowing better why I find some things work and some do not or that the things that I thought were were helping stop helping. Was looking at the wrong thing. The problem is there is VERY little information on what to do about it. I’ve been doing research but I don’t quite understand enough to fully follow what’s known about what to do. Obviously avoiding being food poisoned is likely a key but to be fair I cannot fully control that aspect and it makes eating socially a burden even more than what it was in the past. That and I did read some about possibly Vitamin D helping. I’m working with a newer Dr on this as my regular Dr was pretty unhelpful with my gut issues and the GI Dr has pretty much given up helping me even with this newer information. 🙁
Also, while I have your attention, I was wondering if you might have done any investigation into Endometriosis? The search here on your site came up empty. I have a family member that I recently discovered has this but again my understanding here is pretty limited.
Does B cells have a special gene for the size of antibody .. and how does it work????
please reply its necessary i hope you have the answer
I don’t understand your question.
Hi Chris, I realize this is an old post, but I'm curious about somatic hypermutation and it's relationship to autoimmunity. My daughter has primary lymphedema, and I'm just wondering if there could be an autoimmune connection there. There's very little research on primary lymphedema, and I'm not a scientist, so maybe this is a stupid though entirely. Just curious if you know anything about how autoimmune conditions impact the lymph system I guess.
Anonymous, this is explained in the article. The variations produced are random deviations from the original antibody gene, using enzymes that lead to altered DNA during replication. Then, the various possibilities are honed through selection for compatibility with the antigen. Perhaps if you have more specific questions I can try to answer.
Very informational, but I have a question. How can B cells that a genetically identical make such a variety of antibodies all in response to a single antigen? I'm aware that it starts with protein synthesis and involves splicing, but I'm not sure where to go from there physiology wise.
Thanks man, really intellesting.
Great post. Hope you will have time to cover the subject of why genes that are responsible for major illnesses have not been discovered yet?
This is a great post. It is understandable in a way people can relate. So, does it mean we need not be afraid to be exposed to viruses? Since we create antibodies when we are exposed to pathogens.
Fascinating antibodies. It really helps body fight against diseases. As long as we are healthy and has good immune system.
"… we are attracted to people with different HLA genes than we have precisely so our children will be sure to have a diverse array of them and thus be maximally resistant to pathogens."
Fascinating post Chris. I believe that it is primarily because of the idea in the quote above that attractive faces are average faces:
bit.ly/d7QCrr
Hi Chris,
No criticism. Just trying to give you a bit of good natured ribbing in apreciation -it's obvious you put alot of effort into what you write.
Thanks, …AL. I did stay up kind of late to write it. Did you mean some of it is unclear? If so, could you point out specifics?
Thanks,
Chris
Nice work; reads like you had to get up in the night to write some of the turns of phrase down before mind let you sleep. Now if you can just teach viruses to respect human's "trespassing forbidden" expression.
Hi Gillian,
I just added these two paragraphs:
==========
We must then deal with the problem of autoimmunity. After all, if we can produce trillions of antibodies and respond to practically anything, why don't we usually destroy our own tissues? The first defense is called "receptor editing." B and T cells that react to our own tissues undergo a second round of V(D)J joining in order to further edit the antibody/T cell receptor genes. If they are still-reactive, they will either be destroyed, permanently inactivated, or indefinitely suppressed. The eventual failure of this suppression in some cases is believed to be related to autoimmune disorders.
How do we tell the difference? B and T cells require signals to interpret whether the antigen they bind to is a "good guy" or "bad guy." Scientists debate whether these signals result from certain molecular patterns that look like "pathogens," or instead from general "danger." But when all is in working order, we regulate our vast array of immune potential to use it to our advantage.
======
It's normal to produce antibodies to foods in the gut. However, we don't want this to 1) induce an inflammatory or allergic response, or 2) cause a cross-reaction to our own tissues. And that's where allergies and autoimmunity come in to play. A complicated topic, but nevertheless in general our immune system can run into food antigens without wreaking havoc.
Glad you liked it!
Chris
Truly amazing! And explained in a way I can understand! But what happens when certain foods leak into the blood stream and become antigens? Are we then done for?